The pH dependence is usually due to the side groups of the amino acids. A change in pH changes the protonation pattern and can, in extreme cases, result in protein denaturation. I therefore suspect that your protein stabilizes the enzyme structure, thus keeping it active at sub optimal pH values.
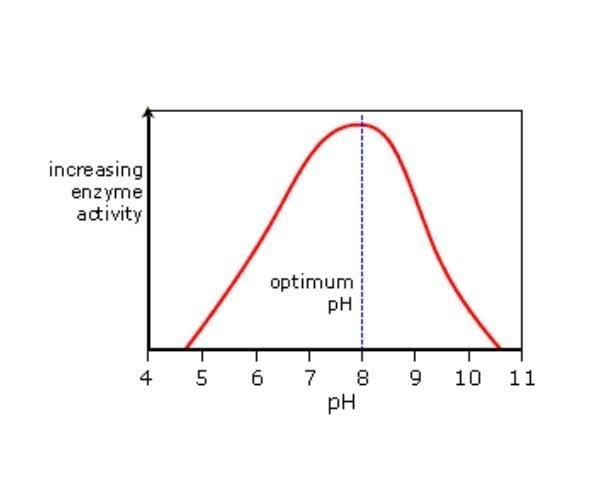
The pH of a solution can have several effects of the structure and activity of enzymes. For example, pH can have an effect of the state of ionization of acidic or basic amino acids. Acidic amino acids have carboxyl functional groups in their side chains. Basic amino acids have amine functional groups in their side chains. If the state of ionization of amino acids in a protein is altered then the ionic bonds that help to determine the 3-D shape of the protein can be altered. This can lead to altered protein recognition or an enzyme might become inactive.
Changes in pH may not only affect the shape of an enzyme but it may also change the shape or charge properties of the substrate so that either the substrate cannot bind to the active site or it cannot undergo catalysis.
In general enzyme have a pH optimum. However the optimum is not the same for each enzyme.
The pH dependence is usually due to the side groups of the amino acids. A change in pH changes the protonation pattern and can, in extreme cases, result in protein denaturation. I therefore suspect that your protein stabilizes the enzyme structure, thus keeping it active at sub optimal pH values.
Souce: NovoPro 2018-03-15
