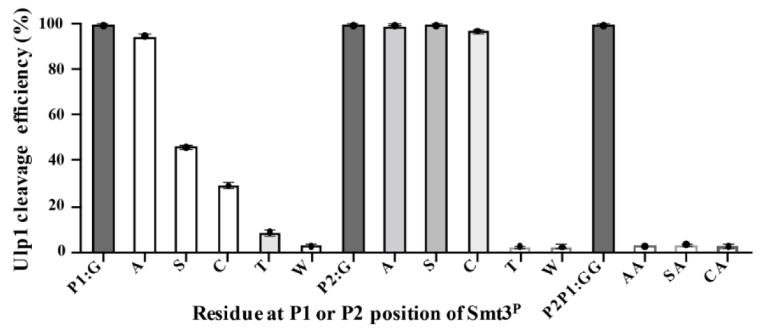
SUMO Protease, also known as Ulp, is a recombinant fragment of ULP1 (Ubl-specific protease 1) from Saccharomyces cerevisiae. It is highly specific for the SUMO protein fusion, recognizing the tertiary structure of SUMO rather than an amino acid. The C-terminal Gly–Gly motif is highly-conserved in all reported SUMO proteins from yeast to human, Ulp1 was sufficient to cleave mutated substrates with preferential activity of Gly > Ala > Ser > Cys at the P1 position and Gly = Ala = Ser = Cys at the P2 position.
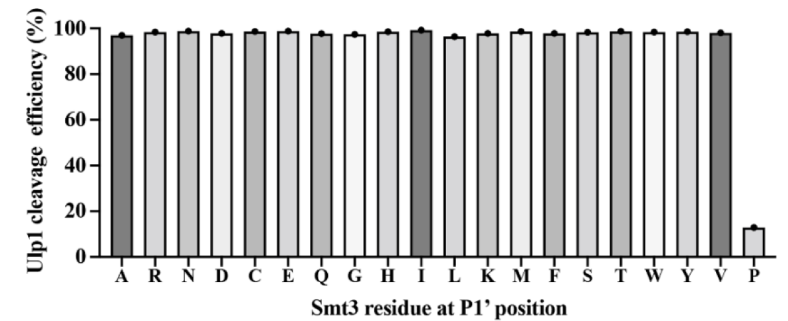
As for Ulp1, its specificity for the P1’ position of Smt3 has been characterized through in vitro and in vivo experiments, demonstrating that mutated Smt3 substrates with any residues at this position can be efficiently cleaved by Ulp1 except Pro.
References:
Zhang F, Zheng H, Xian Y, Song H, Wang S, Yun Y, Yi L, Zhang G. Profiling Substrate Specificity of the SUMO Protease Ulp1 by the YESS–PSSC System to Advance the Conserved Mechanism for Substrate Cleavage. International Journal of Molecular Sciences. 2022; 23(20):12188.
Kretz, C.A.; Tomberg, K.; Van Esbroeck, A.; Yee, A.; Ginsburg, D. High throughput protease profiling comprehensively defines active site specificity for thrombin and ADAMTS13. Sci. Rep. 2018, 8, 2788.
Souce: NovoPro 2023-09-09
