-
Product Name
Anti-Hsc70 Mouse antibody
- Documents
-
Description
Hsc70 Mouse monoclonal antibody
-
Tested applications
WB
-
Species reactivity
Human
-
Isotype
Mouse IgG
-
Preparation
Antigen: Recombinant Protein of Hsc 70.
-
Clonality
Monoclonal
-
Formulation
Purified mouse monoclonal in PBS(pH 7.4) containing with 0.02% sodium azide 50% glycerol.
-
Storage instructions
Store at -20°C
-
Applications
1:1000
-
Validations
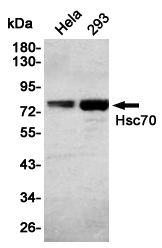
Western blot detection of Hsc70 in Hela,293 cell lysates using Hsc70 Mouse mAb(1:1000 diluted).Predicted band size:70KDa.Observed band size:70KDa.
-
Background
Swiss-Prot Acc.P11142.Molecular chaperone implicated in a wide variety of cellular processes, including protection of the proteome from stress, folding and transport of newly synthesized polypeptides, activation of proteolysis of misfolded proteins and the formation and dissociation of protein complexes. Plays a pivotal role in the protein quality control system, ensuring the correct folding of proteins, the re-folding of misfolded proteins and controlling the targeting of proteins for subsequent degradation (PubMed:21150129, PubMed:21148293, PubMed:24732912, PubMed:27916661, PubMed:23018488). This is achieved through cycles of ATP binding, ATP hydrolysis and ADP release, mediated by co-chaperones (PubMed:21150129, PubMed:21148293, PubMed:24732912, PubMed:27916661, PubMed:23018488). The co-chaperones have been shown to not only regulate different steps of the ATPase cycle of HSP70, but they also have an individual specificity such that one co-chaperone may promote folding of a substrate while another may promote degradation (PubMed:21150129, PubMed:21148293, PubMed:24732912, PubMed:27916661, PubMed:23018488). The affinity of HSP70 for polypeptides is regulated by its nucleotide bound state. In the ATP-bound form, it has a low affinity for substrate proteins. However, upon hydrolysis of the ATP to ADP, it undergoes a conformational change that increases its affinity for substrate proteins. HSP70 goes through repeated cycles of ATP hydrolysis and nucleotide exchange, which permits cycles of substrate binding and release. The HSP70-associated co-chaperones are of three types: J-domain co-chaperones HSP40s (stimulate ATPase hydrolysis by HSP70), the nucleotide exchange factors (NEF) such as BAG1/2/3 (facilitate conversion of HSP70 from the ADP-bound to the ATP-bound state thereby promoting substrate release), and the TPR domain chaperones such as HOPX and STUB1 (PubMed:24318877, PubMed:27474739, PubMed:24121476, PubMed:26865365). Acts as a repressor of transcriptional activation. Inhibits the transcriptional coactivator activity of CITED1 on Smad-mediated transcription. Component of the PRP19-CDC5L complex that forms an integral part of the spliceosome and is required for activating pre-mRNA splicing. May have a scaffolding role in the spliceosome assembly as it contacts all other components of the core complex. Binds bacterial lipopolysaccharide (LPS) and mediates LPS-induced inflammatory response, including TNF secretion by monocytes (PubMed:10722728, PubMed:11276205). Participates in the ER-associated degradation (ERAD) quality control pathway in conjunction with J domain-containing co-chaperones and the E3 ligase STUB1 (PubMed:23990462). Interacts with VGF-derived peptide TLQP-21 (PubMed:28934328).
Related Products / Services
Please note: All products are "FOR RESEARCH USE ONLY AND ARE NOT INTENDED FOR DIAGNOSTIC OR THERAPEUTIC USE"
