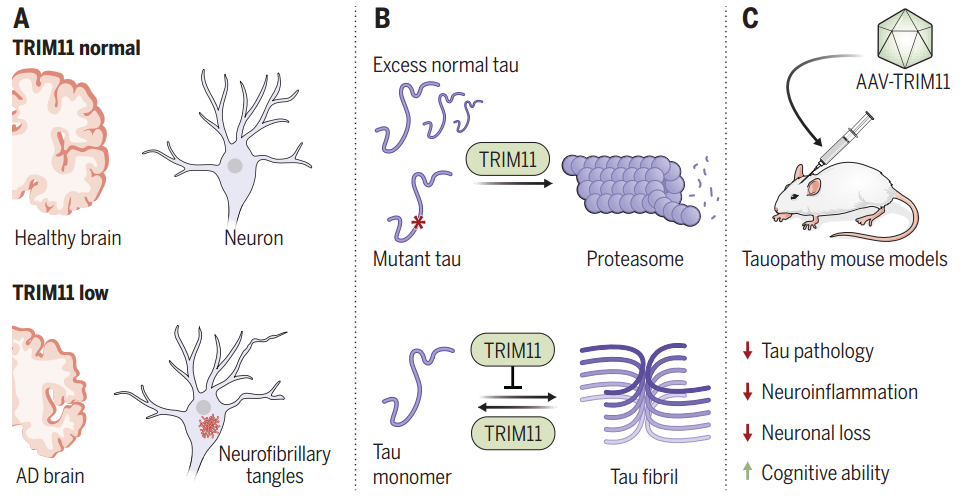
A series of diseases characterized by pathological changes in tau proteins, including Alzheimer's disease (AD), are classified as tauopathies (tauopathies). Their common feature is the presence of intracellular neurofibrillary tangles (NFTs) made up of hyperphosphorylated aggregates of tau protein.
What happens in between, from soluble tau protein monomers to insoluble fiber aggregates?
In both sporadic and hereditary tauopathies, tau deposition occurs in an age-dependent manner, and there may be cytokines that act early in life to inhibit the misfolding and aggregation of tau proteins.
In a new study published today in Science from a team of scientists at the University of Pennsylvania, the researchers identified a triple motif protein, TRIM11, which protects soluble tau monomers from misfolding and abnormal aggregation in several ways. In patients with AD, brain levels of TRIM11 are significantly reduced.
TRIM11 treatment was effective in inhibiting tau pathology and neuroinflammation and improving cognitive and motor performance in three different tauopathy model mice, including the common AD model mouse 3xTg-AD.TRIM11 is likely to be a powerful target for the treatment of tauopathies.
In order to maintain the normal morphology and physiological functions of proteins, there exists an inherent protein quality control system (PQC) in organisms, including degradation pathways to recycle defective proteins and excess normal proteins, molecular chaperones to prevent protein misfolding and aggregation, and depolymerizing enzymes to dissolve pre-existing protein deposits.
Triple motif proteins (TRIM) are a class of proteins characterized by a cyclic structural domain, a B-box motif, and a coiled helix structure; TRIMs are found only in multicellular organisms, and more than 70 have been identified, with some studies suggesting that TRIMs may be involved in PQC.
The researchers focused on TRIM in an attempt to find the key in which regulates tau proteins.
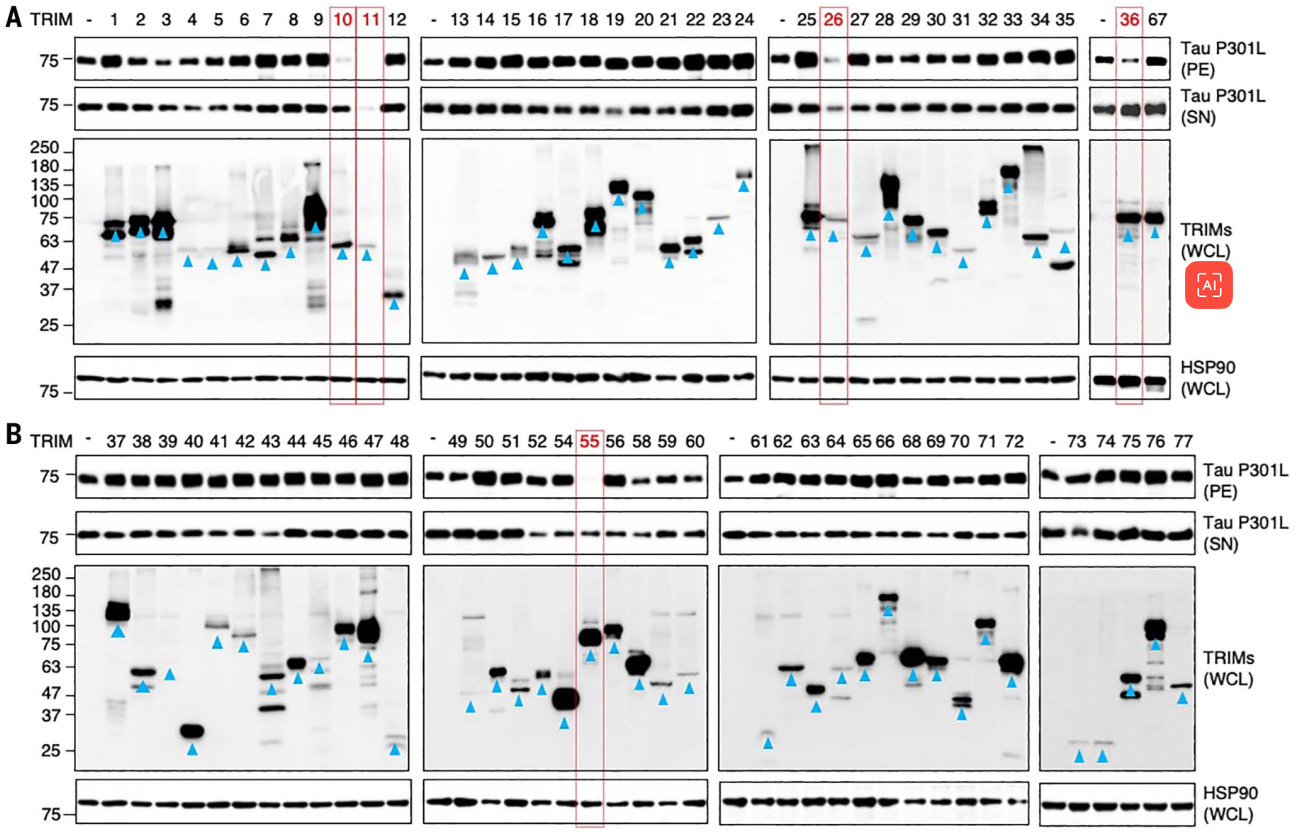
Further analysis revealed that this TRIM11 is really unusual, and it surprisingly has three mechanisms to regulate tau levels.
One, TRIM11 can bind to tau proteins, especially mutant tau or over-phosphorylated tau, and promote its ubiquitination, which ultimately leads to proteasome-mediated degradation.
Second, TRIM11 can exist as a molecular chaperone for tau proteins, which can prevent the misfolding and aggregation of tau proteins.
Third, TRIM11 itself has the ability to break down tau proteins and can dissolve pre-existing tau protein deposits.
So it seems that TRIM11 is really a good move against abnormal tau proteins! So could it be used to treat tauopathy?
The researchers chose three model mice: the PS19 mouse, a common model of tau disease that expresses the common tau mutant P301L, which progressively forms tau deposits; the PS19, which was injected with pre-formed tau protofibrils to exacerbate the disease phenotype; and the 3xTg-AD mouse, which expresses the tau P301L, APP, and PSEN1 mutations simultaneously.
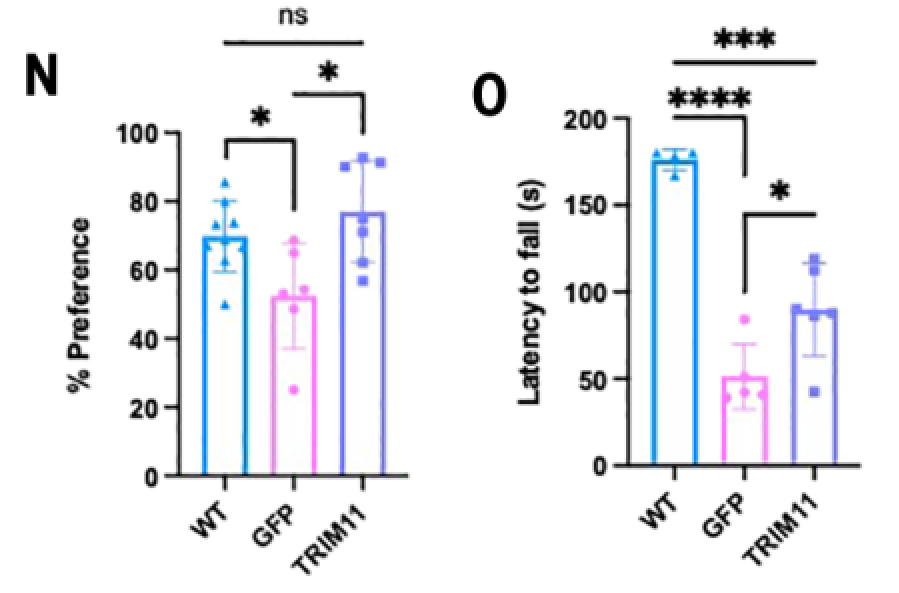
Targeted injection of TRIM11 into the hippocampus of 2.5-month-old PS19 mice via AAV9, and analysis of mouse brain pathology and animal behavior at 10 months of age showed a significant 55% reduction of tau lesions in the brains of treated mice.
Meanwhile, the mice's ability to recognize new objects was similar to that of the wild type, suggesting that the TRIM11 treatment avoided the degradation of long-term memory; the mice's locomotor ability was significantly better than that of the control group, but was still far from the wild type.
Related products:
1. TRIM11 (B30.2/SPRY domain), https://novoprolabs.com/p/human-trim11-recombinant-protein-gst-tag-523904.html
Souce: NovoPro 2023-07-28
